Minimally invasive blood collection techniques as a source of gDNA for genetic studies on turtles and tortoises
Downloads
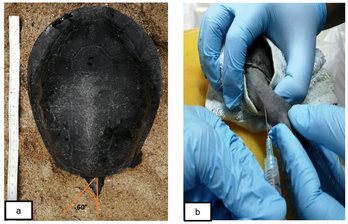
This technical note aims to describe the venipuncture procedure used to collect blood from Southern River terrapins via the subcarapacial venous plexus (SVP) and jugular vein. On uncooperative terrapins, SVP was applied while the jugular vein was reversed. 1.5 ml blood was preserved in 0.5 ml EDTA and stored at -20°C. ReliaPrepTM Blood genomic DNA Miniprep was used to extract DNA. Thermo ScientificTM NanoDrop 2000c was used to determine the concentrations of extracted DNAs. The greatest concentration of DNA is 136.3 g/L, and the highest purity is 1.90. The treatment is safe, minimally invasive, and effective.
Downloads
Avataneo V., D'Avolio A., Cusato J., Cantù M., De Nicolò A. (2019). LC-MS application for therapeutic drug monitoring in alternative matrices. Journal of Pharmaceutical and Biomedical Analysis, 166: 40-51.
Barrows M., McArthur S., Wilkinson R. (2004). Diagnosis in medicine and surgery of tortoises and turtles. Blackwell, Ames, IA. 140 p.
Biase F.H., Franco M.M., Goulart L.R., Antunes R. C. (2002). Protocol for extraction of genomic DNA from swine solid tissues. Genetics and Molecular Biology, 25(3): 313-315.
Bowen B.W., A.L. Bass, A. Garcia-Rodriguez, Bolten A., Diez C.E., van Dam R., Bjorndal K.A., Fer R.J. (1996). Origin of hawksbill turtles in a Caribbean feeding area, as indicated by genetic markers. Ecological Applications. 6: 566-572.
Brajković V., Duvnjak I., FerenÄaković M., Å pehar M., Raguž N., Lukić B., Curik I., Cubric-Curik V. (2018). The effect of DNA quality on the sequencing success of cattle. Journal of Central European Agriculture, 19(4): 804-809.
í‡ilingir F.G., Seah A., Horne B.D., Som S., Bickford D.P., Rheindt F.E. (2019). Last exit before the brink: Conservation genomics of the Cambodian population of the critically endangered southern river terrapin. Ecology and Evolution, 9(17): 9500-9510.
Dutton, P.H. (1996). Methods for collection and preservation of samples for sea turtle genetic studies. In Proceedings of the international symposium on sea turtle conservation genetics. NOAA Technical Memorandum NMFSSEFSC-396. pp: 17-24.
Encalada S.E., Lahanas P.N., Bjorndal K.A., Bolten A.B., Miyamoto M.M., Bowen B.W. 1996. Phylogeography and population structure of the Atlantic and Mediterranean green turtle Chelonia mydas: a mitochondrial DNA control region sequence assessment. Molecular Ecology, 5(4): 473-483.
Fowler M.E. (1995). Restraint and handling of wild and domestic animals, 2nd Ed. Iowa State University Press, USA. 92 p.
García–Feria L.M., Ureña–Aranda C.A., de los Monteros A.E. (2015). Minimally invasive blood sampling method for genetic studies on Gopherus tortoises. Animal Biodiversity and Conservation, 38(1): 31-35.
Gottdenker N.L., Jacobson E.R. (1995). Effect of venipuncture sites on hematologic and clinical biochemical values in desert tortoises (Gopherus agassizii). American Journal of Veterinary Research, 56: 19-21.
Hernández–Divers S.M., Hernandez–Divers S.J., Wyneken J. (2002). Angiographic, anatomic, and clinical technique descriptions of a subcarapacial venipuncture site for chelonians. Journal of Herpetological Medicine and Surgery, 12: 32-37.
Ismail N.A., Ghani N.I.A., Pelf-Nyok C., Manaf L.A., Jamil N.R., Ismail A. (2016). Cross-species microsatellite amplification of River Terrapin (Batagur affinis). Acta Biologica Malaysiana, 5(2&3): 75-79.
Kuchling G. (2012). The reproductive biology of the Chelonia (Vol. 38). Springer Science & Business Media. 224 p.
Mader D.R. (2005). Reptile medicine and surgery, 2nd ed. Elsevier Saunders, St Louis (MO). 1242 p.
Mans C. (2008). Venipuncture techniques in chelonian species. Lab Animal, 37(7): 303-304.
McArthur S., Wilkinson R., Meyer J. (2008). Medicine and surgery of tortoises and turtles. John Wiley and Sons. 579 p.
McDonald, H. S. (1976). Methods for the physiological study of reptiles. Biology of the Reptilia, 5: 19-126.
Owens D.W., Ruiz G. J. (1980). New Methods of Obtaining Blood and Cerebrospinal Fluid from Marine Turtles. Herpetologica, 36(1): 17-20.
Praschag P., Holloway R., Georges A., Paeckert M., Hundsdoerfer A.K., Fritz U. (2009). A new subspecies of Batagur affinis (Cantor, 1847), one of the world's most critically endangered chelonians (Testudines: Geoemydidae). Zootaxa, 2233(1): 57-68.
Randall D., Burggren W., French K. (1997). Eckert Animal Physiology: Mechanisms and Adaptations. W. H. Freeman and Go., New York.
Seutin G., White B.N., Boag, P.T. (1991). Preservation of avian blood and tissue samples for DNA analyses. Canadian Journal of Zoology, 69(1): 82-90.
UC Davis Genome Center (2018). Illumina Library Construction Services – Sample Requirements. Available from: http://dnatech.genomecenter.ucdavis. edu/illumina-library-construction/. Retrieved 2/6/2021.
Wendland L. D., Balbach H., Brown M., Diemer Berish J., Littell L., Clark M. (2009). Handbook on Gopher Tortoise (Gopherus polyphemus). US Army Corps of Engineers.
White P.S., Densmore IIILD. (1992). Mitochondrial DNA isolation. In. Molecular Genetic Analysis of Populations. (ed. Hoelzel AR). Oxford University Press, New York. pp: 29-58.
Copyright (c) 2022 International Journal of Aquatic Biology

This work is licensed under a Creative Commons Attribution 4.0 International License.








